
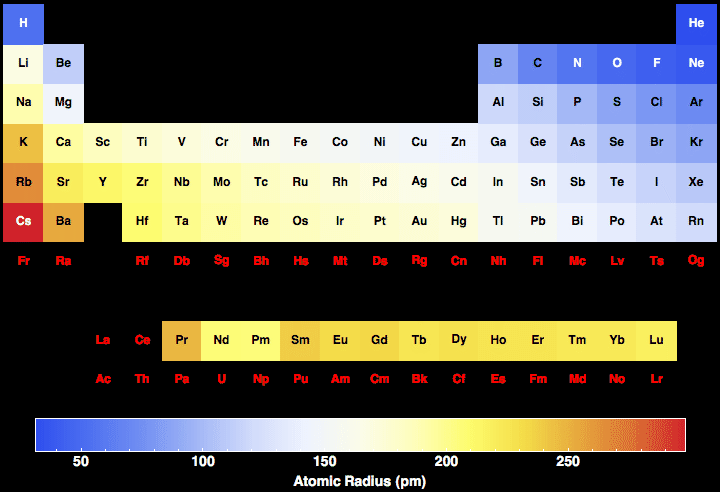


The symmetry properties of the crystal are described by the concept of space groups. The positions of the atoms inside the unit cell are described by the set of atomic positions ( x i, y i, z i) measured from a reference lattice point. The unit cell is represented in terms of its lattice parameters, which are the lengths of the cell edges Lattice Constants (a, b and c) aĪnd the angles between them Lattice Angles (alpha, beta and gamma). As you can see from the diagrams, the same atom could be found to have a different radius. How do you find the atomic radius of an element The radius of an atom can only be found by measuring the distance between the nuclei of two touching atoms, and then halving that distance. The unit Cells repeats itself in three dimensional space to form the structure. The atomic radius of Francium atom is 260pm (covalent radius). Francium has the largest atomic radius, while Helium has the lowest. Atomic Number: 87 Symbol: Fr Group: 1 (Alkali Metals) Number of Protons: 87 Number of Electrons: 87 Number of Neutrons: 136 Atomic Radius: 348pm. The Crystal structure can be described in terms of its unit Cell. Which is the smallest element with the largest atomic radius As can be seen in the figures below, the atomic radius increases from top to bottom in a group, and decreases from left to right across a period. The solid state structure of Francium is Body Centered Cubic. How big is the atomic radius of francium Atomic Radius of Francium The atomic radius of Francium atom is 260pm (covalent radius).


 0 kommentar(er)
0 kommentar(er)
